Rietveld structure refinement to optimize the correlation between cation disordering and magnetic features of CoFe2O4 nanoparticles†
Abstract
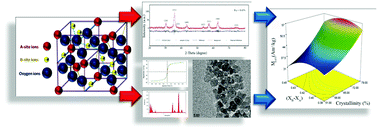
The structural properties of cobalt ferrite nanoparticles have significant effects on their magnetic behavior. In the current study, we thoroughly scrutinized and optimized the structural characteristics of CoFe2O4 nanoparticles and the correlation with their magnetic properties as a function of the synthesis parameters using Rietveld structure refinement. Nanoparticles were synthesized using co-precipitation method based on design of experiments and then characterized using X-ray diffraction, vibrating sample magnetometry, transmission electron microscopy, and energy dispersive X-ray spectroscopy analyses. Based on response surface methodology studies, we identified factors that had an effect on the structural and magnetic features. In order to reach maximum magnetization, the cations distribution was optimized, and the pH amount and reaction temperature were identified as the most influential factors. We observed that the initial cation ratio of Co2+/Fe3+ sharply affected the cations distribution, which was subsequently involved in the different structural characteristics and magnetization of nanoparticles. This can be attributed to the hybrid structure formation and magnetic exchange interactions of cations. Finally, the maximum magnetization was achieved at the optimum cations distribution of (Co0.32 Fe0.68)(Co0.70Fe1.30)O4, where the difference between distributed cobalt cations in tetrahedral and octahedral sites was minimum.


 Please wait while we load your content...
Please wait while we load your content...