Synthesis, photophysical, DFT and photodiode properties of subphthalocyanine–BODIPY dyads
Abstract
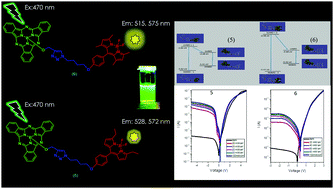
In this study, subphthalocyanine–borondipyrromethene conjugates have been successfully designed and synthesized. The novel compounds were fully characterized by using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, and 1H and 13C NMR spectroscopies. The photophysical properties of the conjugates were investigated by means of absorption and fluorescence spectroscopies in benzene solutions. The results showed that while the absorption spectra of both conjugates remain almost the same, compound 5 has a better fluorescence behavior and higher energy transfer efficiency. To test the effect of the replacement of the methyl group in the conjugates, Density functional theory (DFT) was used to calculate the equilibrium geometries of the ground state for the conjugates. The torsional angle of the BODIPY with reference to Sub-Pc in compound 5 (−125.4°) was different than that in compound 6 (−91.4°). This planarization results in a decrease in the orbital energy of the BODIPY unit in compound 5, making a smaller gap for the energy transfer mechanism between the BODIPY and Sub-Pc unit. In addition, their photodiode properties were tested here. The obtained phototransient current and capacitance results indicated that the devices exhibit both photodiode and photocapacitor properties. Therefore, it can be concluded that the subphthalocyanine–borondipyrromethene conjugate-based photodevices can be used as photodiodes in solar tracking systems.


 Please wait while we load your content...
Please wait while we load your content...