Conformational studies of Ant–Pro motif-incorporated cyclic peptides: gramicidin S and avellanin†
Abstract
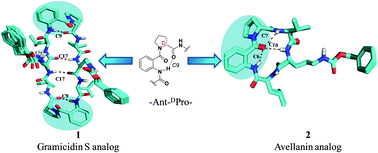
This paper reports conformational changes observed in cyclic bioactive peptides such as gramicidin S and avellanin upon incorporation of a pseudo-β (C9) Ant–DPro turn motif in their structural frameworks. Solution-state studies suggested that a synthetic gramicidin S analog exhibits a β-sheet conformation with C9 and C17 intramolecular hydrogen bonding patterns, while its truncated analog disturbs the β-sheet conformation. Structural details were obtained using a combination of CD studies, X-ray crystal structure studies and nOe-based MD simulation studies.


 Please wait while we load your content...
Please wait while we load your content...