Interaction of Cu(i) with the Met-X3-Met motif of alpha-synuclein: binding ligands, affinity and structural features†
Abstract
The identity of the Cu(I) binding ligands at Met-X3-Met site of AcαS and its role into the affinity and structural properties of the interaction were elucidated by NMR spectroscopy. We provide evidence that the source of ligands for Cu(I) binding to the Met-X3-Met site comes from the N-terminal acetyl group and the Met-1, Asp-2 and Met-5 residues. From the study of site-directed mutants and synthetic peptide models of αS we demonstrated the critical role played by Met-1 and Met-5 residues on the binding affinity of the Cu(I) complex, acting as the main metal anchoring residues. While having a more modest impact in the affinity features of Cu(I) binding, as compared to the Met residues, the N-terminal acetyl group and Asp-2 are important in promoting local helical conformations, contributing to the stabilization of these structures by favoring Cu(I) binding.
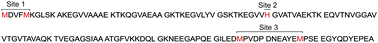
- This article is part of the themed collections: Metallomics Recent Open Access Articles, Metallomics Recent HOT articles and In memory of Silvia Atrian


 Please wait while we load your content...
Please wait while we load your content...