Electrochemical preparation and applications of copper(i) acetylides: a demonstration of how electrochemistry can be used to facilitate sustainability in homogeneous catalysis†
Abstract
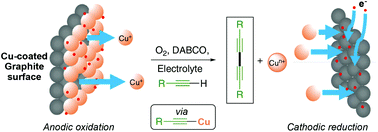
Copper(I) acetylides are important intermediates for many syntheses and have been prepared here electrochemically in an energy efficient manner. These were subsequently employed in simple organic C–C bond forming reactions. We also demonstrate that application of Faraday's laws allows the charge to be calculated so that only the required amount of metal is used. In addition, the application of copper-coated graphite electrodes allows the maximum atom efficiency for this process and even offers a recovery strategy to extract the metal following completion of the reaction.


 Please wait while we load your content...
Please wait while we load your content...