Iridium-catalysed primary alcohol oxidation and hydrogen shuttling for the depolymerisation of lignin†
Abstract
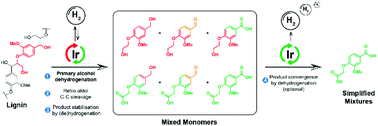
Lignin is a potentially abundant renewable resource for the production of aromatic chemicals, however its selective depolymerisation is challenging. Here, we report a new catalytic system for the depolymerisation of lignin to novel, non-phenolic monoaromatic products based on the selective β-O-4 primary alcohol dehydrogenation with a Cp*Ir-bipyridonate catalyst complex under basic conditions. We show that this system is capable of promoting the depolymerisation of model compounds and isolated lignins via a sequence of selective primary alcohol dehydrogenation, retro-aldol (Cα–Cβ) bond cleavage and in situ stabilisation of the aldehyde products by transfer (de)hydrogenation to alcohols and carboxylic acids. This method was found to give good to excellent yields of cleavage products with both etherified and free-phenolic lignin model compounds and could be applied to real lignin to generate a range of novel non-phenolic monomers including diols and di-acids. We additionally show, by using the same catalyst in a convergent, one-pot procedure, that these products can be selectively channelled towards a single di-acid product, giving much simpler product mixtures as a result.


 Please wait while we load your content...
Please wait while we load your content...