Bio-electrochemical conversion of industrial wastewater-COD combined with downstream methanol synthesis – an economic and life cycle assessment†
Abstract
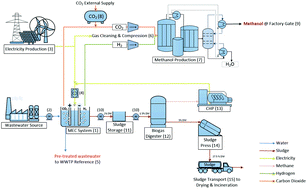
Herein, a techno-economic and environmental performance evaluation (i.e. Life Cycle Assessment (LCA)) of a 45 kW Microbial Electrolysis Cell (MEC) system is presented in the context of industrial wastewater remediation. This system produces H2 and CO2 – suitable for downstream CH3OH synthesis – based on the bio-electrochemical conversion of chemical industry wastewater with an organic content of 3.9 g(COD) L−1. A cost–benefit analysis indicates that the MEC system hardware costs, share of CO2 captured from the MEC and MEC operating current density (i.e. 1.0 mA cm−2) are crucial parameters influencing the total cost and represent areas for potential cost reductions. It was established based on the present study that MEC system operation with renewable electricity leads to H2 production costs of 4–5.7€ kg(H2)−1 (comparable to H2O electrolysis) and CH3OH production costs of 900€ t(CH3OH)−1. At the current CH3OH market prices, however, the production is currently not profitable. In turn, the cost-efficient construction of the MEC system and the use of less expensive materials could lead to improved CH3OH production economics based on this route. Our results indicate that the use of low-cost materials has greater potential with regard to cost reduction compared to reducing the internal resistance and polarization losses via the use of expensive high-performance materials in MEC construction. A complementary LCA of the proposed system, based on a “cradle-to-gate” definition, indicates that waste-based is superior to fossil-based CH3OH production with respect to global warming potential and cumulated fossil energy demand, provided the system is operated with 100% renewable electricity and CO2 sourced only from the MEC. However, with regard to the impact categories Metal Depletion and Freshwater Eutrophication Potential, the system was found to perform less satisfactorily (i.e. in comparison with fossil-based CH3OH production).


 Please wait while we load your content...
Please wait while we load your content...