Visible-light-promoted intramolecular C–H amination in aqueous solution: synthesis of carbazoles†
Abstract
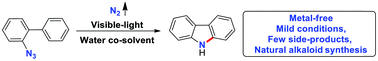
An effective and operationally simple protocol is reported for the synthesis of versatile carbazoles. With water as a co-solvent, visible-light rather than various metals is used to facilitate the conversion of readily available 2-azidobiphenyls under mild conditions. Various functionalized bioactive natural alkaloids, such as glycoborine, clausine C, clausine L, clausine H and clauszoline K, were synthesized efficiently with nitrogen as a sole byproduct. The reaction could be performed in water, acidic or alkaline buffer solutions, showing its potential for applications in biochemistry.


 Please wait while we load your content...
Please wait while we load your content...