Stereochemistry-dependent hydrogen bonds stabilise stacked conformations in jet-cooled cyclic dipeptides: (LD) vs. (LL) cyclo tyrosine–tyrosine†
Abstract
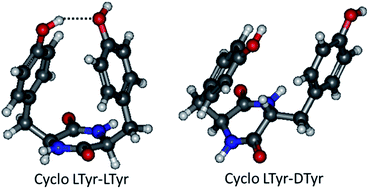
Tyrosine-containing cyclic dipeptides based on a diketopiperazine (DKP) ring are studied under jet-cooled conditions using resonance-enhanced multi-photon ionisation (REMPI), conformer-selective IR-UV double resonance vibrational spectroscopy and quantum chemical calculations. The conformational landscape of the dipeptide containing natural L tyrosine (Tyr), namely c-LTyr–LTyr strongly differs from that of its diastereomer c-LTyr–DTyr. A similar family of conformers exists in both systems, with one aromatic ring folded on the dipeptide DKP ring and the other one extended. Weak NH⋯π and CH⋯π interactions are observed, which are slightly different in c-LTyr–LTyr and c-LTyr–DTyr. These structures are identical to those of LL and LD cyclo diphenylalanine, which only differ from c-Tyr–Tyr by the absence of hydroxyl on the benzene rings. While this is the only conformation observed for c-LTyr–DTyr, c-LTyr–LTyr exhibits an additional form stabilised by the interaction of the two hydroxyls, in which the two aromatic rings are in a stacked geometry. Stereochemical effects are still visible in the radical cation, for which one structure is observed for c-LTyr–DTyr, while the spectrum of the c-LTyr–LTyr radical cation is explained in terms of two co-existing structures.
- This article is part of the themed collection: Quantum effects in small molecular systems


 Please wait while we load your content...
Please wait while we load your content...