Adsorption of selenium(vi) onto nano transition alumina†
Abstract
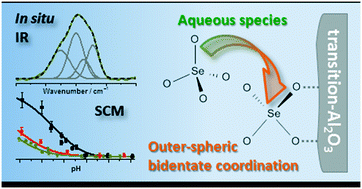
The adsorption of selenium(VI) onto nano transition alumina (γ/δ-Al2O3) was investigated at both macroscopic and molecular levels. The uptake of selenium(VI) was found to decrease upon increasing pH (5–10) and ionic strength (0.01–0.1 mol L−1 NaCl). At the molecular level, in situ attenuated total reflection Fourier-transform infrared (ATR FT-IR) spectroscopy established the predominant formation of a bidentate outer-sphere surface complex throughout the investigated pH range. The acid–base surface properties of transition alumina (surface charge) together with the Se(VI) adsorption edges were successfully described using a 1-pK charge distribution surface complexation model involving one outer-sphere selenium(VI) surface species, namely {(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) AlOH20.5+)2SeO42−} as suggested by the IR studies. Blind predictions of literature data yielded good agreement in particular in NaCl systems. These new spectroscopy based results can be implemented in reactive transport models to enable more consistent and trustworthy prognostic modeling of the environmental fate of selenium(VI).
AlOH20.5+)2SeO42−} as suggested by the IR studies. Blind predictions of literature data yielded good agreement in particular in NaCl systems. These new spectroscopy based results can be implemented in reactive transport models to enable more consistent and trustworthy prognostic modeling of the environmental fate of selenium(VI).


 Please wait while we load your content...
Please wait while we load your content...