Combined high alkalinity and pressurization enable efficient CO2 electroreduction to CO†
Abstract
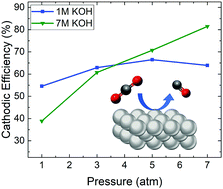
The electroreduction of carbon dioxide (CO2) to carbon monoxide (CO) is a promising strategy to utilize CO2 emissions while generating a high value product. Commercial CO2 electroreduction systems will require high current densities (>100 mA cm−2) as well as improved energetic efficiencies (EEs), achieved via high CO selectivity and lowered applied potentials. Here we report a silver (Ag)-based system that exhibits the lowest overpotential among CO2-to-CO electrolyzers operating at high current densities, 300 mV at 300 mA cm−2, with near unity selectivity. We achieve these improvements in voltage efficiency and selectivity via operation in a highly alkaline reaction environment (which decreases overpotentials) and system pressurization (which suppresses the generation of alternative CO2 reduction products), respectively. In addition, we report a new record for the highest half-cell EE (>80%) for CO production at 300 mA cm−2.


 Please wait while we load your content...
Please wait while we load your content...