Effect of positional isomerism on the spectroelectrochemical response of 3,6-bis(2-pyridyl)-diketopyrrolopyrrolate bridged bis(carbonylhydridoruthenium) compounds†
Abstract
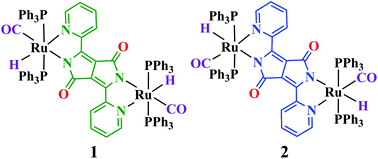
Reaction of 3,6-bis(2-pyridyl)-diketopyrrolopyrrole (H2-BPDPP) with two equivalents of [Ru(H)(CO)(Cl)(PPh3)3] in EtOH produced two symmetrical dinuclear isomers, (μ-BPDPP)[Ru(CO)H(PPh3)2]2, green 1 and blue 2, which could be separated chromatographically and characterised spectroscopically (1H and 31P NMR, IR, and UV-VIS). Isomeric forms of 1 and 2 were authenticated using their single crystal X-ray structures. In addition to the essentially planar bis-chelating bridge BPDPP2− and the mutually trans positioned axial PPh3 ligands in both complexes, compound 1 was established with the CO groups trans to the pyrrolate–N atoms, whereas 2 has the π acceptors CO and pyridine–N situated trans to each other. While the reduction of 1 and 2 proceeds irreversibly at negative potentials, the reversible oxidations at rather low potentials could be monitored by EPR and UV-VIS-NIR absorption measurements. Together with TD-DFT calculations, these results reveal that the primary electron transfers are largely confined to the BPDPP ligand. Despite the bridge centred processes, small differences between the isomers 10/+ and 20/+ were found, affecting e.g. the near infrared absorption of the radical cation species.


 Please wait while we load your content...
Please wait while we load your content...