Exploring the cellular uptake and localisation of phosphorescent rhenium fac-tricarbonyl metallosurfactants as a function of lipophilicity†
Abstract
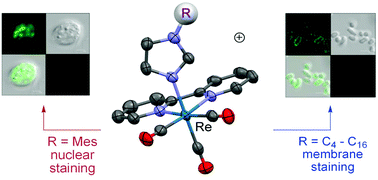
A systematic study of the cellular uptake of emissive complexes as a function of their lipophilicity is presented. Here a series of amphiphilic rhenium fac-tricarbonyl bisimine complexes bearing axial substituted imidazole or thiazole ligands, [Re(bpy)(CO)3(ImCnHm)]+ {n = 1 m = 3 (1+), n = 4 m = 9 (2+), n = 8 m = 17 (3+), n = 12 m = 25 (4+), n = 16 m = 33 (5+), n = 2 m = 3 (6+); bpy = 2,2′-bipyridine, Im = imidazole} and [Re(bpy)(CO)3(L)]+ {L = 1-mesitylimidazole, ImMes (7+), 4,5-dimethylthiazole, dmt (8+) and 4-methyl-5-thiazole-ethanol, mte (9+)} is reported. The X-ray crystal structures of 2+, 8+ and 9+ confirm the geometry and expected distribution of ligands and indicated that the plane of the imidazole/thiazole ring is approximately parallel to the long axis of the bipy ligand. Luminescence studies revealed excellent properties for their use in cell imaging with visible excitation and broad emission profiles. Their uptake in two distinct species has been examined by fluorescence imaging of the diplomonad fish parasite Spironucleus vortens (S. vortens) and rod-shaped yeast Schizosaccharomyces pombe (Schiz. pombe) as a function of their lipophilicity. The uptake of the complexes was highest for the more lipophilic 2+–5+ in both S. vortens and Schiz. pombe in which the long alkyl chain aids in crossing bilipid membranes. However, the increased lipophilicity of longer chains also resulted in greater toxicity. Localisation over the whole cell varied with differing alkyl chain lengths with complex 2+ preferentially locating to the nucleus of S. vortens, 3+ showing enhanced nuclear partitioning in Schiz. pombe, and 4+ for the remaining cell wall bound in the case of S. vortens. Interestingly, complexes of intermediate lipophilicity such as 7+ and 8+ showed reasonable uptake, proved to be non-toxic, and were capable of crossing exterior cell walls and localising in the organelles of the cells.
- This article is part of the themed collection: Molecular metal-containing soft materials


 Please wait while we load your content...
Please wait while we load your content...