Hydrolysis of organometallic and metal–amide precursors: synthesis routes to oxo-bridged heterometallic complexes, metal-oxo clusters and metal oxide nanoparticles
Abstract
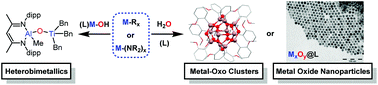
The hydrolysis reaction between Brønsted basic organometallic or metal–amide reagents with Brønsted acidic OH groups from water or metal–hydroxides may act as a controlled stoichiometric strategy for the formation of M–O–M bonds, if careful consideration of reaction conditions is employed. This article explores the utilisation of highly reactive organometallic and metal–amide complexes from across the periodic table as reagents for the synthesis of metal-oxo clusters, oxo-bridged heterobimetallics and metal oxide nanoparticles. Such reactivity typically occurs at low temperatures with the release of hydrocarbon or amine by-products. The impact of ligand coordination, M–C bond strength, M–OH acidity and reaction temperature are discussed.
- This article is part of the themed collection: 2018 Frontier and Perspective articles


 Please wait while we load your content...
Please wait while we load your content...