Comprehensive insight into the support effect of graphitic carbon nitride for zinc halides on the catalytic transformation of CO2 into cyclic carbonates†
Abstract
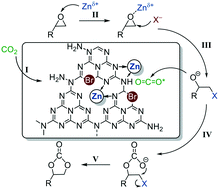
Chemical fixation of CO2 to high-valued chemicals is currently a significant research topic in both the environment and chemistry, and cycloaddition of CO2 with epoxides is regarded as a sustainable route for the manufacture of cyclic carbonates. Homogeneous catalysts including ionic liquids and organic metal complexes suffer from difficulty in catalyst–product separation despite their excellent catalytic activities. In this work, we utilized graphitic carbon nitride (g-C3N4) as a novel support to immobilize zinc halides (ZnX2) through a simple preparation method. Based on the detailed design of the synthesis of ZnX2/g-C3N4, the chemical bonding information of ZnX2 on g-C3N4 was comprehensively investigated by XPS and FT-IR techniques. In addition to activation of CO2, g-C3N4 can anchor zinc halides via interaction between zinc and nitrogen, thereby effectively alleviating potential leaching of zinc halides. As heterogeneous catalysts, ZnX2/g-C3N4 materials showed good catalytic activities in the cycloaddition reactions of CO2 with propylene oxide. Furthermore, a wide range of epoxides can be converted to the corresponding cyclic carbonate with good selectivities (>93%) and moderate conversions (50–88%).


 Please wait while we load your content...
Please wait while we load your content...