Native chemical ligation in protein synthesis and semi-synthesis
Abstract
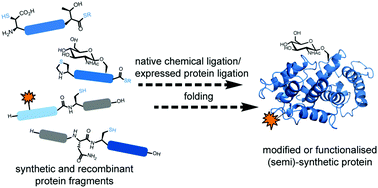
Native chemical ligation (NCL) provides a highly efficient and robust means to chemoselectively link unprotected peptide and protein segments to generate proteins. The ability to incorporate non-proteinogenic amino acids (e.g.D-amino acids or fluorescent labels) and post-translational modifications into proteins by stitching together peptide fragments has driven extremely important developments in peptide and protein science over the past 20 years. Extensions of the original NCL concept (including the development of thiol- and selenol-derived amino acids and desulfurisation and deselenisation methods), improved access to peptide thioesters, and the use of the methodology in combination with recombinantly expressed polypeptide fragments (termed Expressed Protein Ligation, EPL) have helped to further expand the utility of the methodology. Over the past five years, there has been a dramatic increase in the number of proteins that have been accessed by total chemical synthesis and semi-synthesis, including a large range of modified proteins; new records have also been set with regards to the size of proteins that can now be accessed via ligation chemistry. Together these efforts have not only contributed to a better understanding of protein structure and function, but have also driven innovations in protein science. In this tutorial review, we aim to provide the reader with the latest developments in NCL- and EPL-based ligation technologies as well as illustrated examples of using these methods, together with synthetic logic, to access proteins and modified proteins for biological study.
- This article is part of the themed collection: Protein engineering through chemical, genetic and computational manipulation


 Please wait while we load your content...
Please wait while we load your content...