Distal radical migration strategy: an emerging synthetic means
Abstract
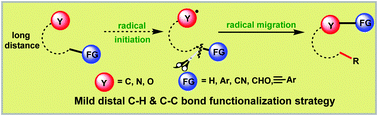
The remote radical migration strategy has gained considerable momentum. During the past three years, we have witnessed the rapid development of sustainable and practical C–C and C–H bond functionalization by means of long-distance 1,n-radical migration (n = 4, 5, 6) events. Its advent brings our chemical community a new platform to deal with the challenging migration transformations and thus complements the existing ionic-type migration protocols. In this review, the recent achievements in distal radical migration triggered C–C and C–H bond functionalization are summarized.


 Please wait while we load your content...
Please wait while we load your content...