Hydrogen bonding from a valence bond theory perspective: the role of covalency†
Abstract
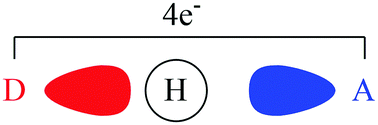
A valence bond theory based method has been developed to decompose hydrogen bond energies into contributions from geometry, electrostatics, polarization and charge transfer. This decomposition method has been carried out for F–H⋯FH, F–H⋯OH2, F–H⋯NH3, HO–H⋯OH2, HO–H⋯NH3, and H2N–H⋯NH3. Localized valence bond self-consistent field (L-VBSCF) and localized breathing orbital valence bond (L-BOVB) calculations were performed at the PBEPBE/aug-cc-pVDZ optimized geometries. It is shown that inclusion of valence bond structures that explicitly include charge transfer account for at least 32% (likely over half) of the hydrogen bond energy of all systems studied, indicating the dominant role of covalency. This is in agreement with calculated bond lengths, geometry deformation energies, and polarization energies. Electrostatic effects were found to play only a minor role in contrast to some widely held ideas regarding the nature of hydrogen bonding.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...