Atomistic determination of the surface structure of Cu2O(111): experiment and theory†
Abstract
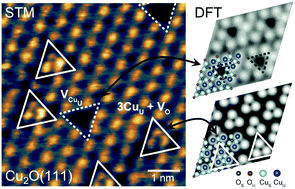
Cuprous oxide (Cu2O) is a promising catalyst for several important reactions. However, the atomic structures of defective Cu2O surfaces, which critically affect the catalytic properties both thermodynamically and kinetically, are not unambiguously characterized. High-resolution scanning tunneling microscopy (STM), combined with density functional theory (DFT) calculations and STM simulations, has been used to determine the atomic structure of the (111) surface of a Cu2O bulk crystal. The single crystal surface, processed by ultrahigh vacuum cleaning and oxygen annealing, shows a (1 × 1) periodicity in the low-energy electron diffraction pattern. The pristine (defect-free) Cu2O(111) surface exhibits a lattice of protrusions with hexagonal symmetry under STM, which is attributed to the dangling bonds of the coordinatively unsaturated copper (CuU) atoms on the surface. Two types of surface atomic defects are also identified, including the CuU vacancy and the oxygen-vacancy-induced local surface restructuring. The electronic structure of this surface measured by dI/dV spectroscopy shows an energy band gap of ∼1.6–2.1 eV. Consistent with dI/dV measurements, DFT calculations identified surface states within the electronic band gap arising from the Cu ions on the surface. Our results provide a clear picture of the pristine and defective Cu2O(111) surface structure in addition to the formation mechanism of the reconstructed surface, paving the way toward studying the site-dependent reactivity of this surface.


 Please wait while we load your content...
Please wait while we load your content...