Confinement of aqueous mixtures of ionic liquids between amorphous TiO2 slit nanopores: electrostatic field induction†
Abstract
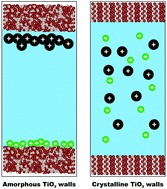
Electrostatic potential in the vicinity of the surface is induced when aqueous mixtures of hydrophobic and hydrophilic ionic liquids (ILs) are confined between a slit nanopore of amorphous but not crystalline TiO2 semiconductors. According to our molecular dynamics (MD) simulations, the extent of ion-pairing lifetime under such nanoscale confinement is substantially lower than its value in the bulk. It becomes still lower when aqueous mixtures of ionic liquid electrolytes are used. Ion–ion correlation is broken completely in the confined dilute aqueous electrolyte systems. The anions and cations of the ILs migrate and accumulate at the opposite amorphous TiO2 electrodes that are separated by 10 nm to arrange a nanosize pore. In contrast, we have shown that the electrostatic interactions between the IL ions are dominant when the electrolyte is confined between anatase (101) TiO2. A similar trend is observed for the inorganic electrolyte system. These findings shed light on the design of new cells for electrochemical applications.


 Please wait while we load your content...
Please wait while we load your content...