Electrolyte solvents for high voltage lithium ion batteries: ion correlation and specific anion effects in adiponitrile†
Abstract
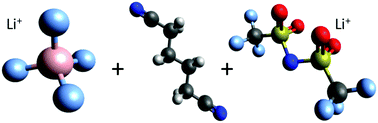
We studied dynamic and structural properties of two lithium conducting salts in the aprotic organic solvent adiponitrile by a combination of atomistic molecular dynamics (MD) simulations, quantum chemical calculations, and experimental findings. The outcomes of our simulations reveal significant differences between both lithium salts, namely lithium tetrafluoroborate (LiBF4) and lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) at various concentrations, which can be mainly attributed to the solvation behavior of the individual anions. The increased tendency of ion complex formation for LiBF4 is reflected by lower values regarding the measured and computed effective ionic conductivities when compared to LiTFSI. All findings highlight the crucial importance of specific anion effects in combination with molecular details of solvation, and advocate the use of adiponitrile as a beneficial solvent in modern lithium ion battery technology with high voltage electrodes.


 Please wait while we load your content...
Please wait while we load your content...