Infrared photodissociation spectroscopy of cold cationic trimethylamine complexes†
Abstract
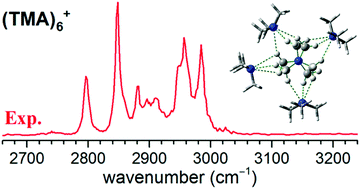
Cryogenic ion-trap infrared photodissociation spectroscopy combined with a dielectric barrier discharge source was constructed to establish the general trends in the stepwise growth motif of trimethylamine (TMA)n+ complexes. The results showed a strong preference for the formation of a stable charge-shared N⋯N type (TMA)2+ ion core over the proton-transferred C⋯HN type ion core, evidencing that the source condition has a remarkable effect on the kinetic stability of isomers. A maximum of four TMA molecules are located perpendicularly to the N⋯N axis of the charge-shared (TMA)2+ ion core. In the n = 7 and 8 clusters, the subsequent two TMA molecules are located at each end of the N⋯N axis of the (TMA)2+ ion core, completing the first coordination shell. Starting at n = 9, the additional TMA molecules form a second solvation shell, and the cluster spectra show similarities to the solution phase spectrum of aqueous TMA.


 Please wait while we load your content...
Please wait while we load your content...