Transport properties and intermolecular interactions in binary mixtures based on the protic ionic liquid ethylimidazolium triflate and ethylene glycol†
Abstract
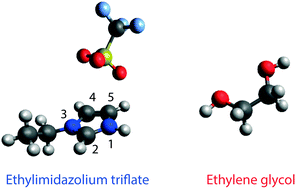
The binary mixture based on the protic ionic liquid (PIL) ethylimidazolium triflate (C2HImTfO) and the diol compound ethylene glycol (EG) has been investigated in the whole composition range from pure PIL to pure EG. At 30 °C the addition of EG increases both the ionic conductivity and the self-diffusivity of the ions. These quantities, however, change at different rates suggesting that the ionicity of the system is composition dependent. This behaviour is explained by means of new intermolecular forces established when a second compound like EG is introduced into the ionic network. More specifically, a complex H-bonded network is formed that involves the –NH group of the cation, the –OH group of EG and the –SO3 group of the anion. This configuration may increase the fluidity of the mixture but not necessarily the ionic dissociation. Moreover, diffusion NMR results indicate the occurrence of local proton dynamics, which arise from a proton exchange between the –NH of the cation and the –OH of EG, providing the requisite for a long-range Grotthuss mechanism of proton transport.


 Please wait while we load your content...
Please wait while we load your content...