Langevin behavior of the dielectric decrement in ionic liquid water mixtures†
Abstract
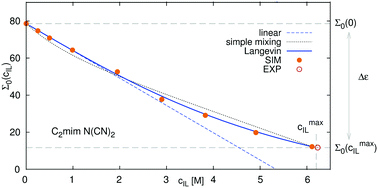
We present large scale polarizable simulations of mixtures of the ionic liquids 1-ethyl-3-methylimidazolium trifluoromethanesulfonate and 1-ethyl-3-methylimidazolium dicyanamide with water, where the dielectric spectra, the ion hydration and the conductivity were evaluated. The dielectric decrement, the depression of the dielectric constant of water upon addition of ions, is found to follow a universal functional of Langevin type. Only three physical properties need to be known to describe the complete range of possible concentrations, namely the dielectric constant of pure water, of pure ionic liquid and the linear slope of the dielectric decrement at low ionic liquid concentrations. Both the generalized dielectric constant, as well as the water contribution to the dielectric permittivity follow the functional dependence. We furthermore find that a scaling of van der Waals parameters upon addition of polarizable forces to the force field is necessary to correctly describe the frequency dependent dielectric conductivity and its contribution to the dielectric spectrum, as well as the static electric conductivity, which is also treated in the framework of a pseudolattice theory.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...