Characterizing the hydrophobic-to-hydrophilic transition of electrolyte structuring in proton exchange membrane mimicking surfaces†
Abstract
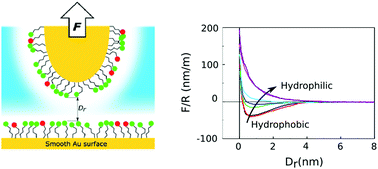
The surface density of charged sulfonic acid head groups in a perfluorosulfonic acid (PFSA) proton exchange membrane determines the hydrophilicity of the ionic channels and is thus critical for the structuring and transport of water and protons. The mechanism through which the head group density affects the structuring of water and ions is unknown, largely due to experimental challenges in systematically varying the density in an appropriate model system resembling the ionic channels. Here, we present a model system for PFSA membrane ionic channels using self-assembled monolayers with a tunable surface density of sulfonic acid and methyl groups to tune surface hydrophilicity. Atomic force microscopy force–distance measurements were used to quantify the hydration forces and deduce the interfacial electrolyte structure. The measured force profiles indicate a pronounced change of the electrolyte layering density at the surface with an unexpectedly sharp hydrophobic-to-hydrophilic transition when the surface shows a contact angle of ∼37°. Using an extended Derjaguin–Landau–Verwey–Overbeek model including the Hydra force, we quantify diffuse double layer charges and characteristic hydration lengths as a function of sulfonic acid group density on the surface. Translating our results to PFSA membranes, these findings have two implications: (1) the density of sulfonic acid head groups can have a dramatic effect on the local solvent structuring of water inside the ionic channels and (2) they support a view where two types of water (solution) exist in PFSA ionic channels – a structured (shell/surface) and a non-structured (bulk) water. This offers an interesting perspective on how different head group densities lead to changes in water and proton transport and macroscopic membrane conductivity properties based on hydration layer characteristics.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...