Aromaticity of acenes: the model of migrating π-circuits
Abstract
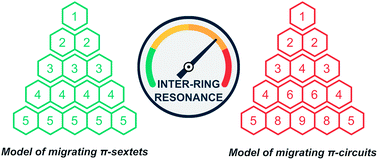
In this work we extend the concept of migrating Clar's sextets to explain local aromaticity trends in linear acenes predicted by theoretical calculations and experimental data. To assess the link between resonance and reactivity and to rationalize the constant-height AFM image of pentacene we used the electron density of delocalized bonds and other functions of the one-electron density from conceptual density functional theory. The presented results provide evidence for migration of Clar's π-sextets and larger circuits in these systems, and clearly show that the link between the theoretical concept of aromaticity and the real electronic structure entails the separation of intra- and inter-ring resonance effects, which in the case of [n]acenes (n = 3, 4, 5) comes down to solving a system of simple linear equations.


 Please wait while we load your content...
Please wait while we load your content...