Site-selective reversible Diels–Alder reaction between a biphenylene-based polyarene and a semiconductor surface†
Abstract
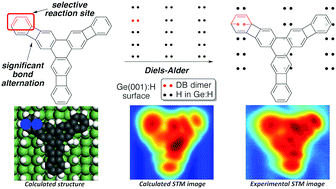
Understanding the mechanisms involved in the covalent attachment of organic molecules to surfaces is a major challenge for nanotechnology and surface science. On the basis of classical organic chemistry mechanistic considerations, key issues such as selectivity and reactivity of the organic adsorbates could be rationalized and exploited for the design of molecular-scale circuits and devices. Here we use tris(benzocyclobutadieno)triphenylene, a singular Y-shaped hydrocarbon containing antiaromatic cyclobutadienoid rings, as a molecular probe to study the reaction of polycyclic conjugated molecules with atomic scale moieties, dangling-bond (DB) dimers on a hydrogen-passivated Ge(001):H surface. By combining molecular design, synthesis, scanning tunneling microscopy and spectroscopy (STM/STS) and computational modeling, we show that the attachment involves a concerted [4+2] cycloaddition reaction that is completely site-selective and fully reversible. This selectivity, governed by the bond alternation induced by the presence of the cyclobutadienoid rings, allows for the control of the orientation of the molecules with respect to the surface DB-patterning. We also demonstrate that by judicious modification of the electronic levels of the polycyclic benzenoid through substituents, the reaction barrier height can be modified. Finally, we show that after deliberate tip-induced covalent bond cleavage, adsorbed molecules can be used to fine tune the electronic states of the DB dimer. This power to engineer deliberately the bonding configuration and electronic properties opens new perspectives for creating prototypical nanoscale circuitry.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...