Effect of conformational flexibility on photophysics of bis-coumarins†
Abstract
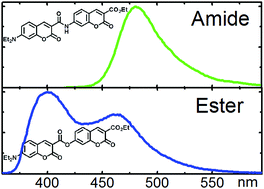
The fluorescence intensity of bis-coumarins linked via CONH and COO functionalities are shown to exhibit a strong dependance on solvent polarity. The presence of the intramolecular hydrogen bond between the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O oxygen atom of coumarin and amide-NH moieties reduces the number of thermodynamically populated conformations in both ground and electronically excited states and an anti-arrangement of coumarin units is favored. Additionally, the rigidity of the linker raises the barrier to the conical intersection with the ground state, and in non-polar solvents strong fluorescence is observed. Although changing the CONH linking position from 3–7′ to 3–6′, does not remarkably affect the photophysics, replacement with a flexible ester linker allows the molecule a greater degree of conformational freedom due to the absence of the intramolecular hydrogen bonding interaction. The photophysical effect of this is the appearance of two fluorescence bands, the relative intensity and spectral positions of which are sensitive to the environment. Theoretical explorations of the excited-state potential energy surfaces performed with the aid of the ADC(2) ab initio electronic structure theory method revealed an exceptional wealth of concomitant photophysical processes. In particular, two channels for radiationless deactivation of the excited state were found; the first is related to the inter-ring twist of the coumarin units, and the second is associated with the excited-state intramolecular proton-transfer (ESIPT) from the CONH linker to the coumarin core.
O oxygen atom of coumarin and amide-NH moieties reduces the number of thermodynamically populated conformations in both ground and electronically excited states and an anti-arrangement of coumarin units is favored. Additionally, the rigidity of the linker raises the barrier to the conical intersection with the ground state, and in non-polar solvents strong fluorescence is observed. Although changing the CONH linking position from 3–7′ to 3–6′, does not remarkably affect the photophysics, replacement with a flexible ester linker allows the molecule a greater degree of conformational freedom due to the absence of the intramolecular hydrogen bonding interaction. The photophysical effect of this is the appearance of two fluorescence bands, the relative intensity and spectral positions of which are sensitive to the environment. Theoretical explorations of the excited-state potential energy surfaces performed with the aid of the ADC(2) ab initio electronic structure theory method revealed an exceptional wealth of concomitant photophysical processes. In particular, two channels for radiationless deactivation of the excited state were found; the first is related to the inter-ring twist of the coumarin units, and the second is associated with the excited-state intramolecular proton-transfer (ESIPT) from the CONH linker to the coumarin core.


 Please wait while we load your content...
Please wait while we load your content...