Sub-μL measurements of the thermal conductivity and heat capacity of liquids†
Abstract
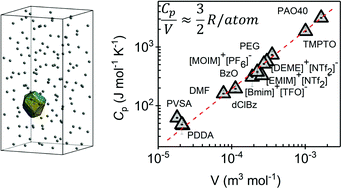
We present the analysis of the thermal conductivity, κ, and heat capacity, Cp, of a wide variety of liquids, covering organic molecular solvents, ionic liquids and water–polymer mixtures. These data were obtained from ≈0.6 μL samples, using an experimental development based on the 3ω method, capable of the simultaneous measurement of κ and Cp. In spite of the different type and strength of interactions, expected in a priori so different systems, the ratio of κ to the sound velocity is approximately constant for all of them. This is the consequence of a similar atomic density for all these liquids, notwithstanding their different molecular structures. This was corroborated experimentally by the observation of a Cp/V ≈ 1.89 × 106 J K−1 m−3 (≈3R/2 per atom), for all liquids studied in this work. Finally, the very small volume of the sample required in this experimental method is an important advantage for the characterization of systems like nanofluids, in which having a large amount of the dispersed phase is sometimes extremely challenging.


 Please wait while we load your content...
Please wait while we load your content...