Comment on “Isomerization of the methoxy radical revisited: the impact of water dimers” by B. Bandyopadhyay et al., Phys. Chem. Chem. Phys., 2016, 18, 27728 and “Isomerization of methoxy radical in the troposphere: competition between acidic, neutral and basic catalysts” by P. Kumar, B. Bandyopadhyay et al., Phys. Chem. Chem. Phys., 2017, 19, 278
Abstract
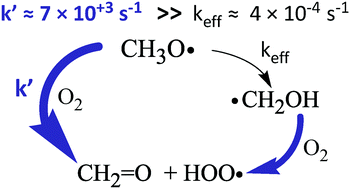
In two recent papers Bandyopadhyay and co-workers studied how atmospheric trace gases catalyze the isomerization of methoxy radical (CH3O˙) to hydroxymethyl radical (˙CH2OH). Their second paper extensively discussed the altitude dependence of this catalyzed isomerization. Unfortunately, they did not compare their computed isomerization rates with the abundant kinetic data on the long-established fate of CH3O˙: reaction with O2. This Comment shows that the fastest rate they compute for catalyzed isomerization is over one million times slower than the O2 reaction at all altitudes considered in those papers. Furthermore, we argue that, even if the reaction CH3O˙ → ˙CH2OH were to occur, it would not have any atmospheric consequence. This is because the near-exclusive atmospheric fate of both ˙CH2OH and CH3O˙ is reaction with O2 to produce CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O + HOO.
O + HOO.


 Please wait while we load your content...
Please wait while we load your content...