Importance of protein flexibility in molecular recognition: a case study on Type-I1/2 inhibitors of ALK†
Abstract
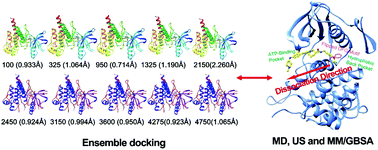
Anaplastic lymphoma kinase (ALK) has been regarded as a promising target for the therapy of various cancers. A large number of ALK inhibitors with diverse scaffolds have been discovered, and most of them belong to Type-I inhibitors that only occupy the ATP-binding pocket. Recently, we reported a series of novel and potent Type-I1/2 inhibitors of ALK with the 1-purine-3-piperidinecarboxamide scaffold, which can bind to both the ATP-binding site of ALK and the adjacent hydrophobic allosteric pocket. In this study, the binding mechanisms of these Type-I1/2 ALK inhibitors were elucidated by multiple molecular modeling techniques. The calculation results demonstrate that the ensemble docking based on multiple protein structures and the MM/PB(GB)SA calculations based on molecular dynamics (MD) simulations yield better predictions than conventional rigid receptor docking (Glide, Surflex-Dock, and Autodock Vina), highlighting the importance of incorporating receptor flexibility in the predictions of binding poses and binding affinities of Type-I1/2 ALK inhibitors. Furthermore, the umbrella sampling (US) simulations and MM/GBSA binding free energy decomposition analyses indicate that Leu1122, Leu1198, Gly1202 and Glu1210 in the hinge region and Glu1197, Ile1171, Phe1174, Ile1179, His1247, Ile1268, Asp1270 and Phe1271 in the allosteric pocket of ALK are the key residues for determining the relative binding strength of the studied inhibitors. Besides, we found that the most potent inhibitor (001-017) tends to form stronger transient interactions with residues along the dissociation channel due to the high electronegativity of its bulky 4-(trifluoromethoxy) phenylamine tail. As a whole, both the stronger binding affinity and the higher energetic barrier (which may prolong the drug-target residence time) of 001-017 contribute to its excellent anti-proliferation activity against ALK-positive cancer cells.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...