Promiscuous hydrogen in polymerising plasmas
Abstract
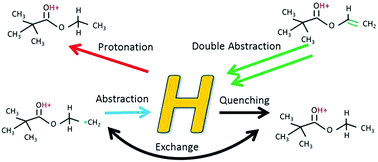
Historically, there have been two opposing views regarding deposition mechanisms in plasma polymerisation, radical growth and direct ion deposition, with neither being able to fully explain the chemistry of the resultant coating. Deposition rate and film chemistry are dependent on the chemistry of the plasma phase and thus the activation mechanisms of species in the plasma are critical to understanding the relative contributions of various chemical and physical routes to plasma polymer formation. In this study, we investigate the roles that hydrogen plays in activating and deactivating reactive plasma species. Ethyl trimethylacetate (ETMA) is used as a representative organic precursor, and additional hydrogen is added to the plasma in the form of water and deuterium oxide. Optical emission spectroscopy confirms that atomic hydrogen is abundant in the plasma. Comparison of the plasma phase mass spectra of ETMA/H2O and ETMA/D2O reveals that (1) proton transfer from hydronium is a common route to charging precursors in plasma, and (2) hydrogen abstraction (activation) and recombination (deactivation) processes are much more dynamic in the plasma than previously thought. Consideration of the roles of hydrogen in plasma chemistry may then provide a more comprehensive view of deposition processes and bridge the divide between the two disparate schools of thought.


 Please wait while we load your content...
Please wait while we load your content...