Conformational sampling of the intrinsically disordered dsRBD-1 domain from Arabidopsis thaliana DCL1†
Abstract
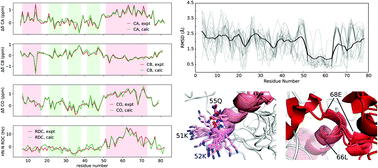
DCL1 is the ribonuclease that carries out miRNA biogenesis in plants. Substrate pri-miRNA recognition by DCL1 requires two double stranded RNA binding domains located at the C-terminus of the protein. We have previously shown that the first of these domains, DCL1-A, is intrinsically disordered and folds upon binding pri-miRNA. Integrating NMR and SAXS data, we study here the conformational landscape of free DCL1-A through an ensemble description. Our results reveal that secondary structure elements, corresponding to the folded form of the protein, are transiently populated in the unbound state. The conformation of one of the dsRNA binding regions in the free protein shows that, at a local level, RNA recognition proceeds through a conformational selection mechanism. We further explored the stability of the preformed structural elements via temperature and urea destabilization. The C-terminal helix is halfway on the folding pathway in free DCL1-A, constituting a potential nucleation site for the final folding of the protein. In contrast, the N-terminal helix adopts stable non-native structures that could hinder the correct folding of the protein in the absence of RNA. This description of the unfolded form allows us to understand details of the mechanism of binding-induced folding of the protein.


 Please wait while we load your content...
Please wait while we load your content...