A computational study of the catalytic aerobic epoxidation of propylene over the coordinatively unsaturated metal–organic framework Fe3(btc)2: formation of propylene oxide and competing reactions†
Abstract
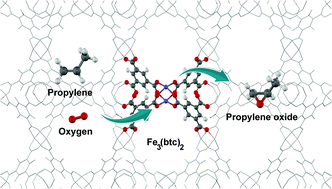
The aerobic epoxidation of propylene over the metal–organic framework Fe3(btc)2 (btc = 1,3,5-benzentricarboxylate) as catalyst has been investigated by means of density functional calculations. The mechanisms of the reaction towards propylene oxide, carbonylic products (acetone and propanal) and a pi-allyl radical were investigated to assess the efficiency of Fe3(btc)2 for the selective formation of propylene oxide. Propylene oxide and carbonylic products are formed on Fe3(btc)2 by proceeding via propyleneoxy intermediates in the first step. Subsequently, the intermediates can then either be transformed to propylene oxide by way of ring closure of the intermediate or to the carbonylic compounds of propanal and acetone via 1,2-hydride shift. The results show that the formation of propylene oxide is favored over the formation of carbonylic products mainly due to the activation barriers being 2–3 times smaller. The activation barriers for the formation of the propyleneoxy intermediates on the Fe3(btc)2 catalyst for the first and second reaction cycle are also lower than the barriers obtained for the formation of the pi-allyl radical that acts as the precursor to combustion products. On the basis of these computational results, we therefore expect a high catalytic selectivity of the Fe3(btc)2 catalyst with respect to the formation of propylene oxide. We also compared the catalytic activities of Fe3(btc)2 and Cu3(btc)2. The activation energy of the rate-determining step is almost 2 times lower for Fe3(btc)2 than that for Cu3(btc)2, due to a larger charge transfer from the catalytic site to the O2 molecule in the case of Fe3(btc)2.


 Please wait while we load your content...
Please wait while we load your content...