The influence of anion chemistry on the ionic conductivity and molecular dynamics in protic organic ionic plastic crystals†
Abstract
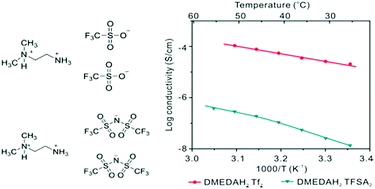
Proton conductors are widely used in different electrochemical devices including fuel cells and redox flow batteries. Compared to conventional proton conducting polymer membranes, protic organic ionic plastic crystal (POIPC) is a novel solid-state proton conductor with high proton conductivity even under anhydrous conditions. In this work, different organic protic salts based on the same parent di-functional cation with different anions were synthesized and characterized. It is found that the di-protonated cation plays an important role in defining the thermal properties, leading to stronger plastic crystal behavior and a higher melting point. Static solid-state NMR and the synchrotron XRD results show that the di-protonated cation allows greater dynamics in the crystal in contrast to the mono-protonated counterparts. The 1-(N,N-dimethylammonium)-2-(ammonium)ethane triflate ([DMEDAH2][Tf]2) has the highest ionic conductivity of 1.1 × 10−4 S cm−1 at 50 °C, whereas the bis(trifluoromethanesulfonyl)amide counterpart [DMEDAH2][TFSA]2 has the lowest ionic conductivity (2.8 × 10−7 S cm−1 at 50 °C) with no measureable mobile ion component at this temperature. The fraction of mobile species is significantly suppressed in the TFSA containing salts as against the Tf systems.


 Please wait while we load your content...
Please wait while we load your content...