The influence of the size and symmetry of cations and anions on the physicochemical behavior of organic ionic plastic crystal electrolytes mixed with sodium salts†
Abstract
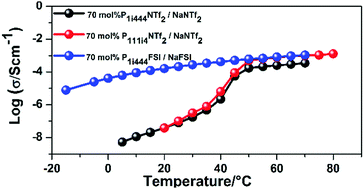
The phase behaviour, ionic conductivity, electrochemical stability and diffusion coefficients of mobile components in three organic ionic plastic crystals (OIPCs): triisobutylmethylphosphonium bis(fluorosulphonyl)amide (P1i444FSI), triisobutylmethylphosphonium bis(trifluromethanesulphonyl)amide (P1i444NTf2) and trimethylisobutylphosphonium bis(trifluoromethanesulphonyl)amide (P111i4NTf2) are compared to study the effect of the anions and cations on phase behaviour and dynamics. The FSI-based OIPC shows lower melting point and higher conductivity values most likely because of the higher degree of charge distributions and weaker ion–ion interactions compared to NTf2 anion-based OIPCs. Cyclic voltammetry of electrolytes consisting of these OIPCs with 70 mol% sodium salt incorporated indicates stable sodium plating/stripping behaviour at 70 and 50 °C for all samples. The magnitude of the peak currents, however, are much higher for the FSI-based electrolyte.


 Please wait while we load your content...
Please wait while we load your content...