Unravelling the nature of glyphosate binding to goethite surfaces by ab initio molecular dynamics simulations†
Abstract
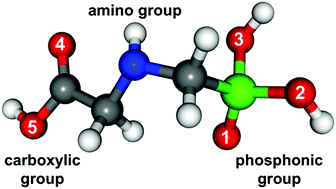
Investigation of the interaction between glyphosate (GLP) and soil minerals is essential for understanding GLP's fate in the environment. Whereas GLP–goethite binding has been discussed extensively, the impact of water as well as of different goethite surface planes has not been studied yet. In this contribution, periodic density functional theory-based molecular dynamics simulations are applied to explore possible binding mechanisms for GLP with three goethite surface planes (010, 001, and 100) in the presence of water. The investigation included several binding motifs of monodentate (M) and bidentate (B) type. It was found that the binding stability increases in the order M@001 < M@010 < (2O + 2Fe) B@100 < M@100 < (1O + 2Fe) B@001 < (2O + 1Fe) B@010. This behavior has been traced to the presence of intramolecular H-bonds (HBs) in GLP as well as intermolecular HBs between GLP and water, GLP and goethite, and water and goethite. These interactions are accompanied by proton transfer from GLP to water and to goethite, and from water to goethite as well as water dissociation at the goethite surface. Further, it was observed that the OH− species can replace the adsorbed GLP at the goethite surface, which could explain the well-known drastic drop in GLP adsorption at high pH. The present results highlight the role of water in the GLP–goethite interaction and provide a molecular level perspective on available experimental data.


 Please wait while we load your content...
Please wait while we load your content...