Hydrated and dehydrated Ca-coordination polymers based on benzene-dicarboxylates: mechanochemical synthesis, structure refinement, and spectroscopic characterization†
Abstract
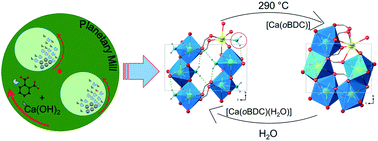
A series of Ca-based coordination polymers were prepared mechanochemically by milling Ca(OH)2 with phthalic acid (H2oBDC), isophthalic acid (H2mBDC), and terephthalic acid (H2pBDC). The hydrated compounds [Ca(oBDC)(H2O)] (1), [Ca(mBDC)(H2O)3.4] (2), and [Ca(pBDC)(H2O)3] (3) were prepared for the first time via mechanochemical routes. The refined structures were validated by extended X-ray absorption data. The new dehydrated compound [Ca(oBDC)] (1-H2O), obtained after the thermal post-treatment of 1 in a reversible phase transition process, was determined ab initio based on the powder X-ray diffraction (PXRD) data. The materials were thoroughly characterized using elemental analysis, thermal analysis, and spectroscopic methods: magic-angle spinning NMR and attenuated total reflection-infrared spectroscopy. The specific surface areas and sorption properties of the hydrated and dehydrated samples were determined using the isotherms of gas sorption and dynamic vapor sorption measurements.


 Please wait while we load your content...
Please wait while we load your content...