Regioselective C–H alkenylation of imidazoles and its application to the synthesis of unsymmetrically substituted benzimidazoles†
Abstract
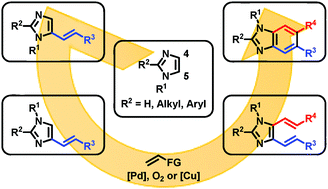
A palladium-catalyzed C–H alkenylation of imidazoles has been developed. High C5 selectivity was achieved for C2-unsubstituted and C2-substituted imidazoles using oxygen and copper(II) acetate, respectively, as oxidants. The obtained products were applied to benzannulation through a sequence involving transposition of N-alkyl groups to give C4-alkenyl imidazoles, alkenylation, thermal 6π-electrocyclization, and oxidation, affording unsymmetrically substituted benzimidazoles.
- This article is part of the themed collections: Celebrating the 75th Anniversary of the Korean Chemical Society (KCS) and 2018 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...