Enantioselective synthesis of chiral oxazolines from unactivated ketones and isocyanoacetate esters by synergistic silver/organocatalysis†
Abstract
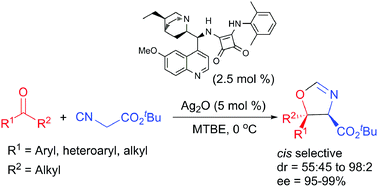
A multicatalytic approach that combines a bifunctional Brønsted base–squaramide organocatalyst and Ag+ as Lewis acid has been applied in the reaction of unactivated ketones with tert-butyl isocyanoacetate to give chiral oxazolines bearing a quaternary stereocenter. The formal [3+2] cycloaddition provided high yields of the corresponding cis-oxazolines with good diastereoselectivity and excellent enantioselectivity, being applied to aryl–alkyl and alkyl–alkyl ketones.


 Please wait while we load your content...
Please wait while we load your content...