Rh(iii)-Catalyzed ortho-C-(sp2)–H amidation of ketones and aldehydes under synergistic ligand-accelerated catalysis†
Abstract
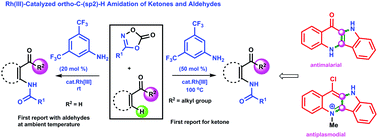
Rh(III)-Catalyzed ortho-C–H amidation of ketones and aldehydes under cooperative metal organocatalysis has been utilized for synthesizing various ortho-amidocarbonyl analogs, and the reaction for the aldehyde proceeds at ambient temperature. The aniline derivative 3,5-bis(trifluoromethyl)aniline promotes the amidation reaction via a transient imine directing group. The efficient amidation agent is dioxazolone. The synthetic utility has been demonstrated in the synthesis of quindolinone alkaloids.


 Please wait while we load your content...
Please wait while we load your content...