Rational design of new cyclic analogues of the antimicrobial lipopeptide tridecaptin A1†
Abstract
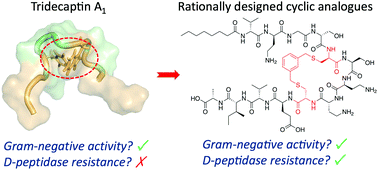
Non-ribosomal peptides (NRPs) are a rich source of antibiotic candidates. However, it was recently discovered that resistance to NRPs can be mediated by D-stereoselective peptidases. The tridecaptins, a class of NRPs that selectively target Gram-negative bacteria, are degraded by the D-peptidase TriF. Through analysis of a solution NMR structure of tridecaptin A1, we have rationally synthesized new cyclic tridecaptin analogues that retain strong antimicrobial activity and are resistant to TriF.


 Please wait while we load your content...
Please wait while we load your content...