Visible-light promoted fluoroalkylselenolation: toward the reactivity of unsaturated compounds†
Abstract
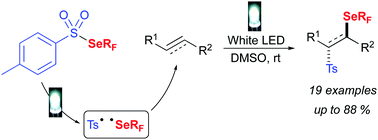
The reactivity of fluoroalkylselenotoluenesulfonates with unsaturated substrates is explored herein. The direct activation of these shelf-stable reagents under visible light allows the double functionalisation of alkenes or alkynes efficiently, leading to a wide range of β-fluoroalkylselenolated sulfones. Mechanistic investigations have been undertaken supporting the formation of radical intermediates.
- This article is part of the themed collections: Selenium & Tellurium chemistry at the beginning of the 3rd millennium: a celebration of ICCST and Fluorine Chemistry


 Please wait while we load your content...
Please wait while we load your content...