Reductive coupling of two aldehydes to unsymmetrical E-alkenes via phosphaalkene and phosphinate intermediates†
Abstract
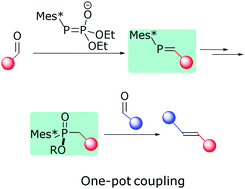
Stilbenes with push–pull electronics are directly accessible from an electron-rich and an electron-deficient benzaldehyde in a novel reductive aldehyde cross-coupling reaction. The one-pot procedure is enabled by the oxidation of a transient phosphinite to the corresponding phosphinate which exhibits sufficient reactivity towards deactivated aldehydes.


 Please wait while we load your content...
Please wait while we load your content...