Re-designing the α-synuclein tetramer†
Abstract
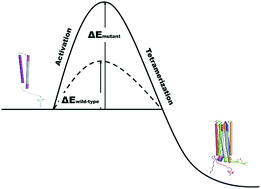
A computationally re-designed molecular loop optimizes helical packing of α-synuclein monomers to seal the aggregation-resistant low-weight tetramer, a key target for Parkinson's disease. Helical monomers are pushed into active conformations during supramolecular assembly, and familial missense mutations double the energy barrier to tetramerization, preserving the pool of potentially amyloidogenic monomers.


 Please wait while we load your content...
Please wait while we load your content...