Unveiling the redox-active character of imidazolin-2-thiones derived from amino-substituted N-heterocyclic carbenes†
Abstract
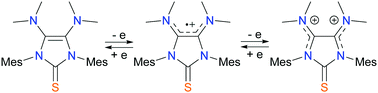
IMes-derived thioureas in which the imidazolyl ring is directly substituted by one or two dimethylamino groups are redox-active, exhibiting one and two oxidized states, respectively. The structure, stability, and electronics of the oxidized species are investigated, emphasizing the decisive role of the amino substituents.


 Please wait while we load your content...
Please wait while we load your content...