Copper-catalysed cross-coupling of alkyl Grignard reagents and propargylic ammonium salts: stereospecific synthesis of allenes†
Abstract
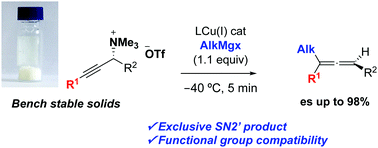
Herein we describe a robust and practical method to prepare enantiomerically enriched trisubstituted allenes using alkyl Grignard reagents and bench stable propargylic ammonium salts. Excellent yields as well as regio- and stereoselectivities are observed. Our conditions provide a solution to the allene racemization, which has been a long-standing problem when using Grignard reagents.


 Please wait while we load your content...
Please wait while we load your content...