Visible-light-induced alkoxyl radical generation for inert chemical bond cleavage/functionalization
Abstract
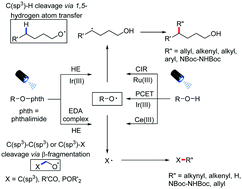
Inert chemical bond cleavage and functionalization reactions provide expedient synthetic routes and are fundamental in organic synthesis. Alkoxyl radicals enable inert chemical bond cleavages by hydrogen atom transfer and β-fragmentation reactivity; however their further synthetic transformations and functional group compatibility are limited by traditional alkoxyl radical generation methods. Recently, visible-light-induced alkoxyl radical generation methods have emerged and led to new advancements in inert chemical bond cleavage reactions and subsequent functionalization, with excellent chemoselectivity and functional group compatibility. In this Feature Article, the generation of alkoxyl radicals by different visible-light-induced methods and their common or distinct reactivity are discussed, which are categorized by C(sp3)–H, C(sp3)–C(sp3), and C(sp3)–X bond cleavages and subsequent transformations.


 Please wait while we load your content...
Please wait while we load your content...