Formal water oxidation turnover frequencies from MIL-101(Cr) anchored Ru(bda) depend on oxidant concentration†
Abstract
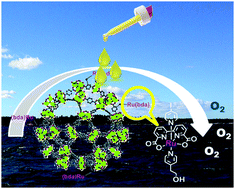
The molecular water oxidation catalyst [Ru(bda)(L)2] has been incorporated into pyridine-decorated MIL-101(Cr) metal–organic frameworks. The resulting MIL-101@Ru materials exhibit turnover frequencies (TOFs) up to ten times higher compared to the homogenous reference. An unusual dependence of the formal TOFs on oxidant concentration is observed that ultimately arises from differing amounts of catalysts in the MOF crystals being active.


 Please wait while we load your content...
Please wait while we load your content...