A synthesis-enabled relative stereochemical assignment of the C1–C28 region of hemicalide†
Abstract
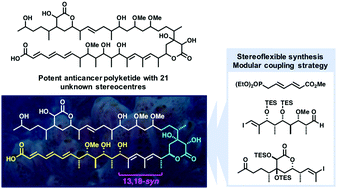
Through synthesising both candidate diastereomers of a model C1–C28 fragment of the potent cytotoxic marine polyketide hemicalide, an assignment of the relative configuration between the C1–C15 and C16–C26 regions has been achieved. By detailed NMR comparisons with the natural product, the relative stereochemistry between these two 1,6-related stereoclusters is elucidated as 13,18-syn rather than the previously proposed 13,18-anti relationship. A flexible and modular strategy using an advanced C1–C28 ketone fragment 22 is outlined to elucidate the remaining stereochemical features and achieve a total synthesis.


 Please wait while we load your content...
Please wait while we load your content...